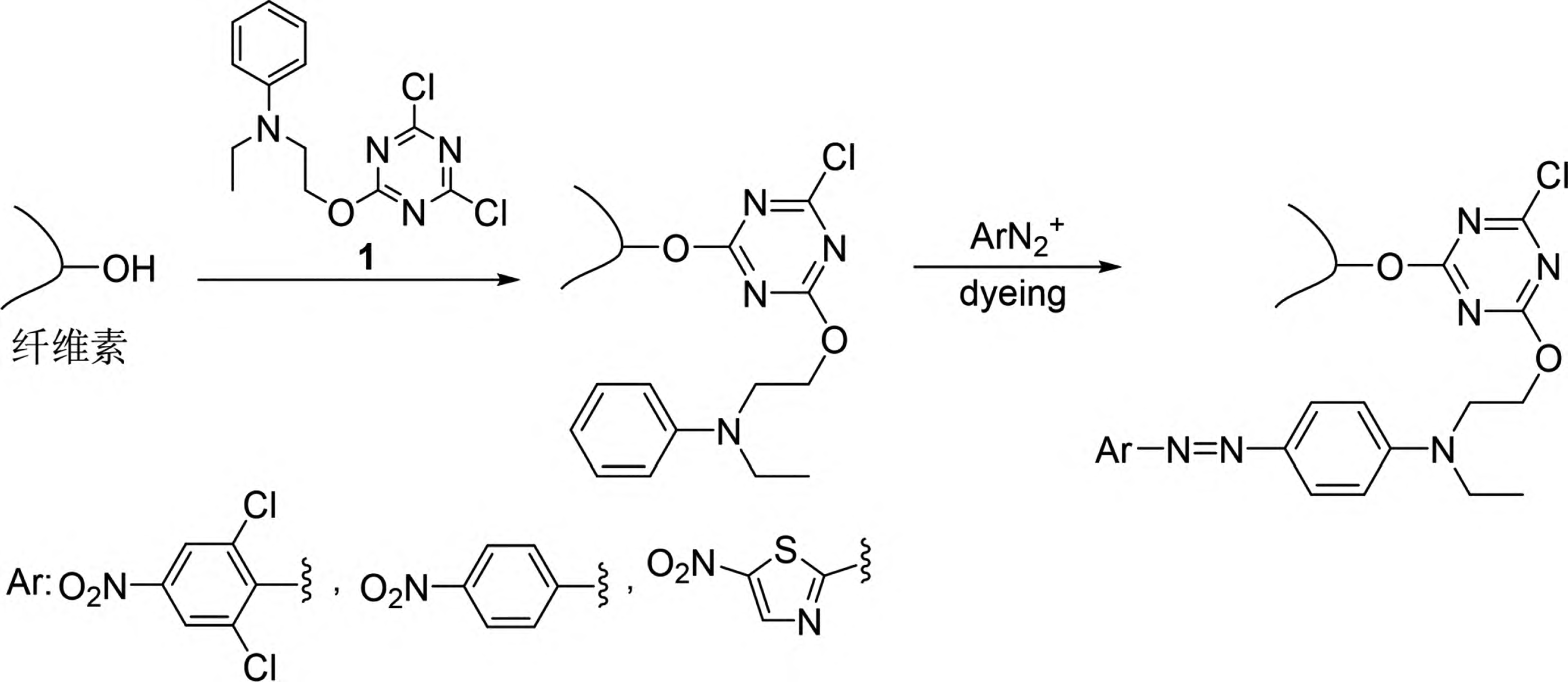
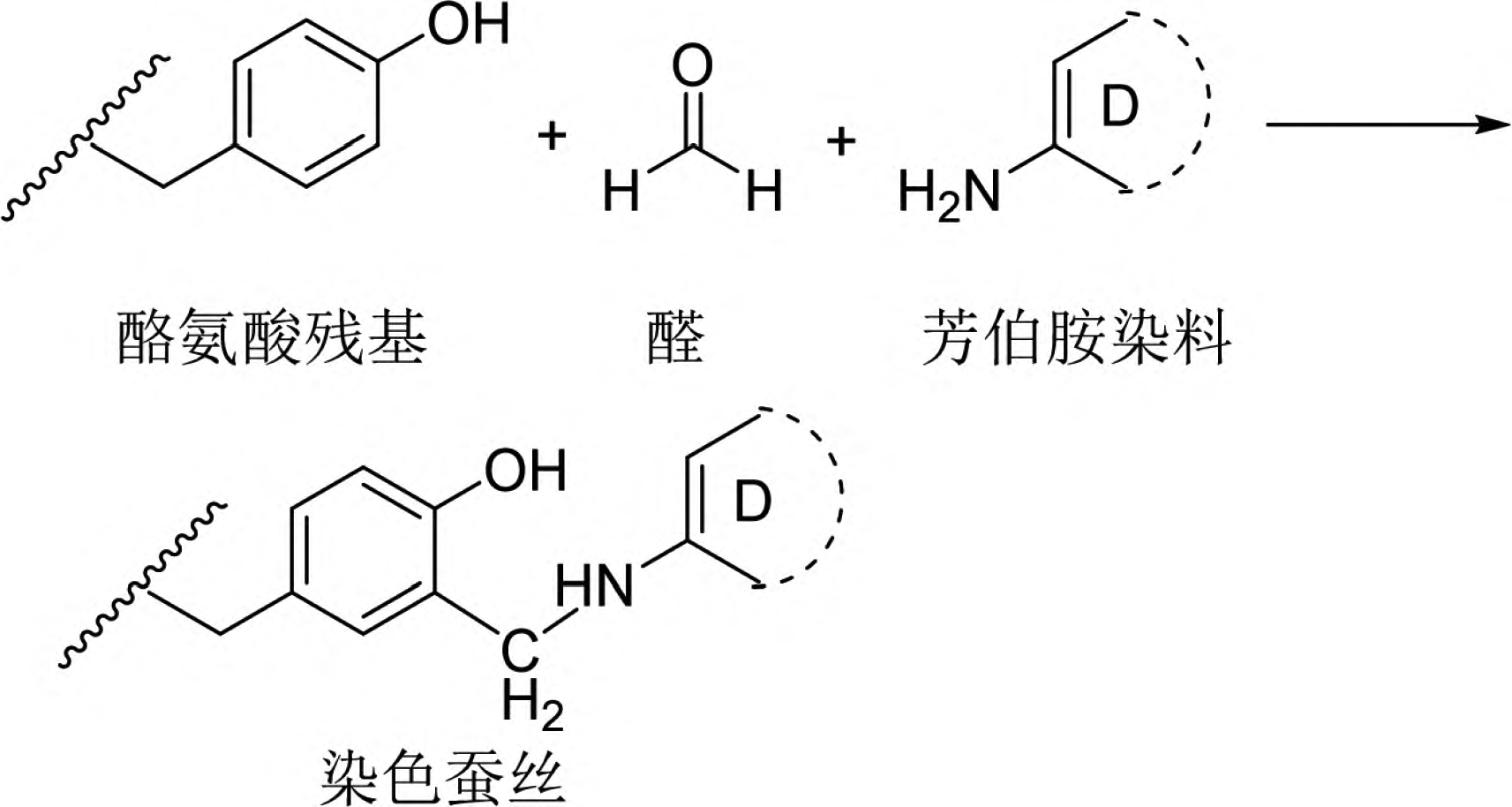
4. Dyeing Method Based on the Maillard Reaction
The Maillard reaction is a non-enzymatic browning reaction widely present in food processing. It involves the interaction between carbonyl compounds (reducing sugars) and amino compounds (amino acids and proteins), undergoing complex processes such as rearrangement, polymerization, and condensation, ultimately forming brown or even black macromolecular compounds known as melanoidins. This reaction, also referred to as the carbonyl-amino reaction, has long been utilized in the food industry for controlling the color of products in fields like baking, coffee processing, meat processing, fragrance production, and brewing. Dyeing professionals have speculated whether the Maillard reaction can be applied to color fibers containing amino groups [25-30].
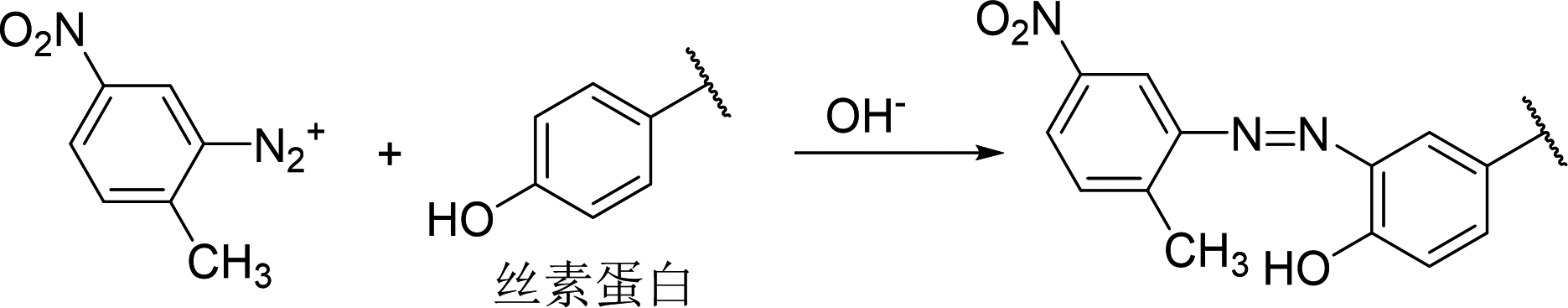
Ohe et al. confirmed that fibers containing amino groups, such as wool, silk, and nylon, can acquire color through the Maillard reaction [25]. Among them, wool exhibited the best coloring effect (Figure 8). For instance, when wool, silk, and nylon were immersed in a 0.1 mol/L xylose aqueous solution at 100°C for 4 hours, wool achieved a K/S value of 3.8, while silk and nylon only reached K/S values of 0.48 and 0.86, respectively [25]. Therefore, research on the Maillard reaction dyeing of wool has been the most extensively reported. The dyeing method using the Maillard reaction has several advantages, such as simple operation, good wash fastness on wool, inexpensive and safe raw materials, easy wastewater treatment, and no need to prepare dyes. However, there are also significant drawbacks, including slow reaction rates that require a long time for color change to occur, limited color variety that is not very vibrant, and poor light fastness. Currently, related expansion research focuses on optimizing the Maillard reaction dyeing method in terms of raw materials and processes to achieve practical application. Additionally, researchers are applying the Maillard reaction principle to graft antimicrobial or antioxidant substances onto wool fibers, imparting more functionality to the dyeing method.
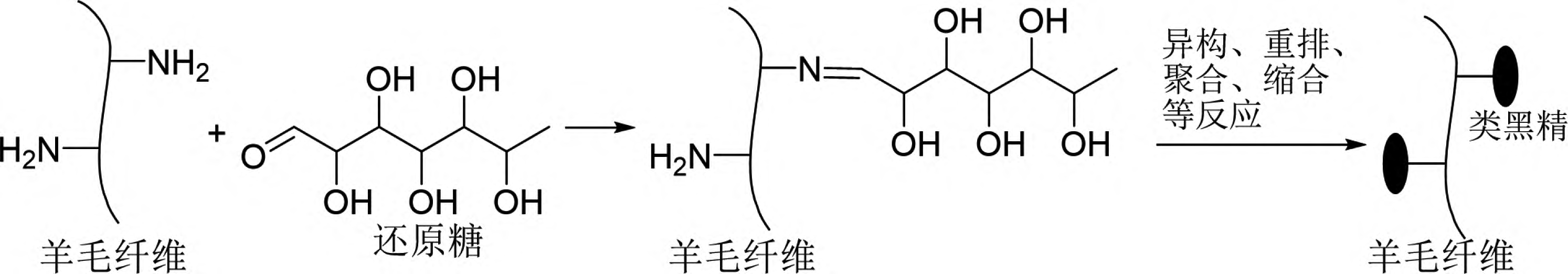
Figure 8 Schematic diagram of the Maillard reaction principle of reducing sugar on wool dyeing
5. Dyeing Method Based on Carbine Chemical Reactions
Carbine-type dyes and their insertion reaction principle for dyeing synthetic fibers.
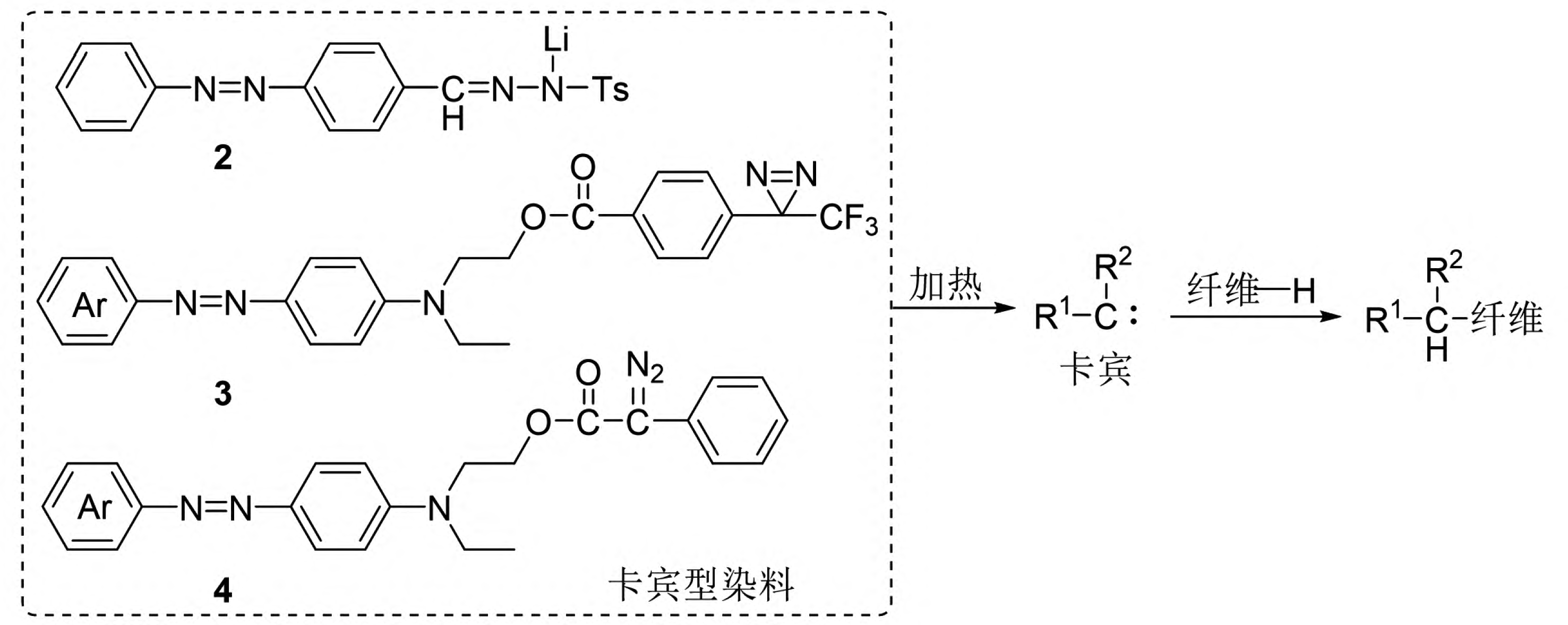
Figure 9 Schematic diagram of the insertion reaction principle of carbene dyes for dyeing synthetic fibers
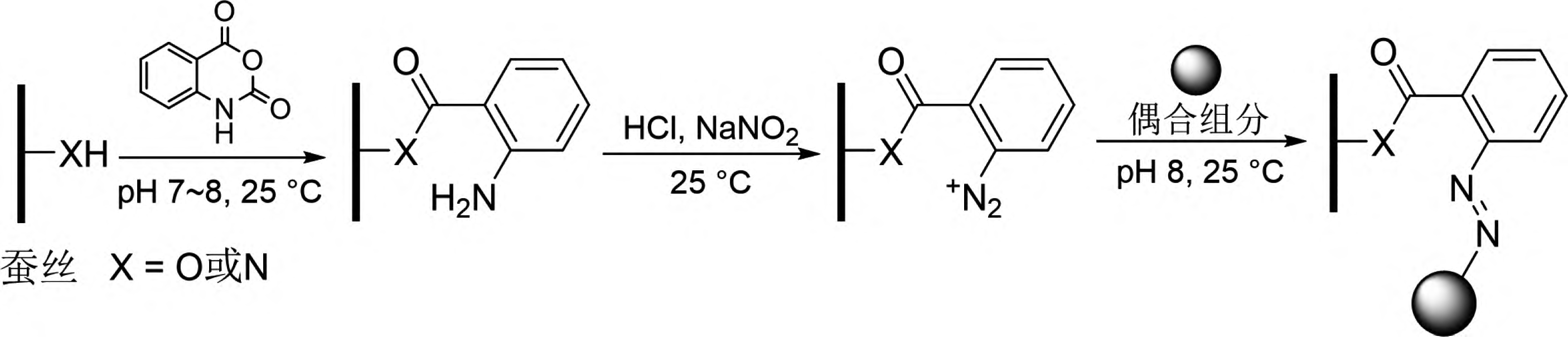
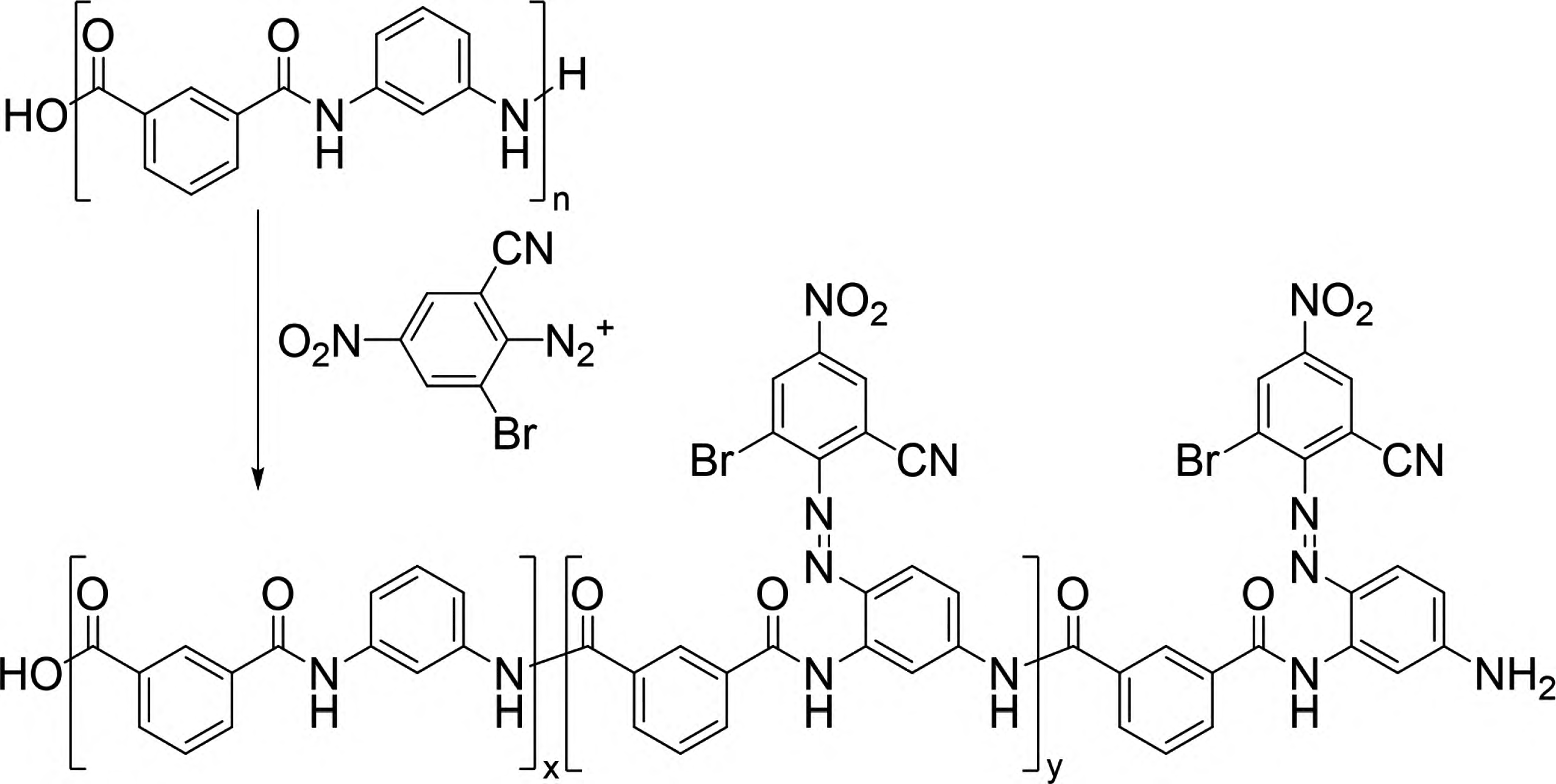
Carbines refer to a class of substances where the carbon atom has only six valence electrons. The simplest carbine is the methylene group (∶CH2). Carbines are highly reactive and can undergo various types of chemical reactions, such as intramolecular rearrangements, dimerization, and intermolecular reactions like insertion and addition. The insertion reaction of carbines refers to the reaction in which a carbine inserts itself into C-H, N-H, or O-H bonds, forming new C-C, C-N, or C-O bonds. Based on this, Lee et al. introduced the para-toluenesulfonylhydrazone structure into dye molecules and treated them with n-butyllithium to obtain a new reactive dye (Compound 2 in Figure 9) [31,32]. This dye, when subjected to high-temperature treatment (140°C), releases the para-toluenesulfonylhydrazone unit to form a carbine intermediate, which can then react chemically with C-H bonds on synthetic fibers such as polypropylene, thus firmly binding the dye chromophore to the synthetic fiber. This new type of reactive dye can be referred to as a carbine-type dye. However, carbine-type dyes based on para-toluenesulfonylhydrazone structures have several drawbacks, including inconvenient dye synthesis, poor dyeing performance, low atom economy, and environmentally harmful by-products.
Jiang Hua and Zhao Tao et al. designed and developed a series of carbine-type dyes based on the structure of bisacridine (Compound 3 in Figure 9) [33-36]. These dyes, upon high-temperature treatment, form carbines by releasing nitrogen molecules, significantly improving atom economy and producing more environmentally friendly by-products. Their application is highly effective, with the dyes reacting chemically with various synthetic fibers such as polyester, polypropylene, aramid, acrylic, nylon, and spandex to achieve dyed synthetic fiber fabrics with high color fastness. Jiang Hua et al. further explored the feasibility of using α-phenyl diazonium ester structures as carbine precursors, and the developed dye (Compound 4 in Figure 9) exhibited excellent dyeing fastness on spandex [37]. Carbine-type dyes and their corresponding dyeing methods are still in the preliminary exploration stage, with limited dye structures, unclear dyeing fastness mechanisms, and a need for further research into their structure-activity relationships to establish a solid foundation for the practical application of these dyes.
6. Other Reactive Dyeing Methods for Spandex
After polymerization, polyurethane may contain trace amounts of isocyanate groups. Based on this, Hanna proposed a reactive dyeing method for polyurethane elastomeric materials, with the reaction mechanism shown in Figure 10 [38].
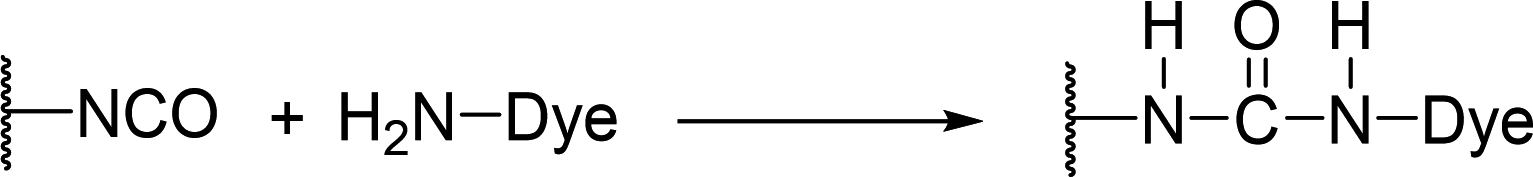
Figure 10. Reaction Mechanism of Amino Dyes with Polyurethane Materials
This method uses amino-containing dyes to dye freshly made polyurethane materials. The amino group in the dye reacts with the residual isocyanate groups on the polyurethane, thus binding the dye to the polyurethane material. The dyeing procedure is as follows: the polyurethane material is placed in a perchloroethylene solution of the dye, with a bath ratio of 1:50 and dye concentration of 1 g/L, followed by boiling at 121°C for 60 minutes to obtain the dyed material. The author used C.I. Disperse Black 1 for dyeing experiments, and the dyed polyurethane’s color remained intact after washing with organic solvents such as ethanol, dioxane, and acetone. Since isocyanate groups are highly reactive and sensitive to water, this dyeing method cannot be performed in aqueous solutions.
Mishukova et al. used p-dimethylamino cinnamaldehyde as an example to dye spandex, with the reaction mechanism shown in Figure 11 [39].
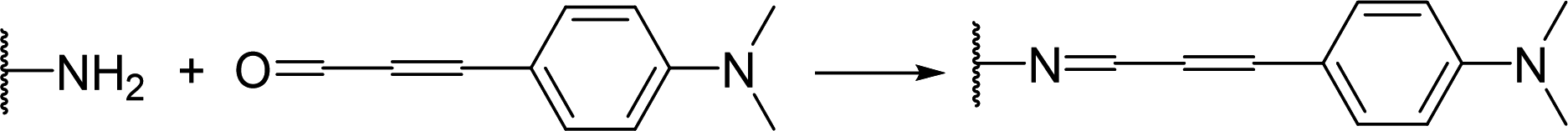
Figure 11. Reaction Mechanism of p-Dimethylamino Cinnamaldehyde with Spandex
This method utilizes the aldehyde group to react with the amino groups on spandex fibers, forming colored Schiff base derivatives on the fibers to achieve color. The dyeing procedure is as follows: set spandex at 5 g, bath ratio of 1:20, aldehyde concentration from 0.1% to 3% (o.w.f.), pH < 2, and dyeing at 100°C for 45 minutes. The maximum wavelength of the reflectance curve of the dyed spandex is at 540 nm, and when the aldehyde concentration is 1%, the color depth of the dyed spandex reaches over 20.
7. Conclusion
The development of new dyeing methods based on chemical reactions requires a thorough consideration of both the characteristics of the chemical reactions and the fiber structure. The role of chemical reactions is mainly to form covalent bonds, thereby firmly attaching the dye chromophore to the fiber. Some chemical reactions can also form new conjugated systems (such as coupling reactions, Maillard reactions, etc.). These new dyeing methods, based on chemical reactions, offer new approaches for obtaining fibers with high color fastness. However, compared to existing dyeing methods, these new methods often still have unsatisfactory aspects in terms of effectiveness, efficiency, or cost. Additionally, some dyeing methods cause noticeable damage to the fibers, and these issues need to be addressed through further research to find appropriate solutions. Furthermore, as the concept of environmental sustainability gains importance, the development of new dyeing methods must take into account the impact of reagents and production waste on human health and the environment, while still meeting the high-quality demands of consumers.
References
[1] Zheng, J. H., Shao, J. Z., Liu, J. Q. A preliminary study on the distribution of tyrosine in silk fibroin proteins. Journal of Textile Science, 2001, 22(6): 351-353.
[2] Chen, W. G., Wang, Z. Q., Cui, Z. H., et al. Study on coloration of silk based on coupling reaction with a diazonium compound. Fibers and Polymers, 2014, 15(5): 966-970.
[3] Yang, H. W., Wang, Z. Q., Xu, J. J., et al. Coupling modification and process control of tyrosine residues in silk fibroin. Journal of Textile Science, 2018, 39(9): 102-108.
[4] Jiang, H., Cai, J. F., Cui, Z. H., et al. Structure-activity relationship of aromatic amine diazonium salts for silk coupling dyeing. Silk, 2019, 56(6): 1-5.
[5] Jiang, H., Zhang, Z. H., Cai, J. F., et al. Diazotization-coupling dyeing of silk fabric with aniline-based dyes and process control. Journal of Textile Science, 2019, 40(11): 100-105.
[6] Guo, Q., Shang, Z. Z., Chen, W. G., et al. In situ generation of azo dyes on silk fibroin through three-step chemical modification. Dyes and Pigments, 2024, 228: 112245.
[7] Jiang, H., Song, J. X., Cui, Z. H., et al. Coupling coloration of meta-aramid fabric utilizing diazonium salts from weakly basic aromatic amines. Coloration Technology, 2024, 140: 75-90.
[8] Lu, L. L., Song, J. X., Hua, K. W., et al. Coupling dyeing process and performance of meta-aramid fibers. Dyeing and Dyeing, 2023, 60(6): 14-18.
[9] Fujian Fiber Testing Center. A method for qualitative identification and application of meta-aramid and para-aramid fibers: CN114689540A. 2022-07-01.
[10] Ding, G. Q., Jiang, H. Coupling coloration of cotton fiber modified with an aniline derivative. Cellulose, 2024, 31: 1311-1328.
[11] Bhate, P. M., Devi, R. V., Dugane, R., et al. A novel reactive dye system based on diazonium salts. Dyes and Pigments, 2017, 145: 208-215.
[12] Hu, Q., Cai, J. F., Jiang, H., et al. Reactive dyeing process and mechanism of aniline-based dyes on cotton fabrics. Journal of Zhejiang Sci-Tech University, 2018, 39(5): 533-538.
[13] Hande, P. R., Badve, P. P., Dugane, R. G., et al. A three-bath process for dyeing cotton with bis-azo bi-functional reactive dyes based on diazonium salts. Fibers and Polymers, 2020, 21(12): 2827-2835.
[14] Hande, P., Kulkarni, K. S., Adivarekar, R. V., et al. A process for dyeing cotton with direct dyes possessing primary aromatic amino groups furnishing wash fastness exhibited by reactive dyes. Coloration Technology, 2022, 138: 248-254.
[15] Olyaei, A., Sadeghpour, Mahdieh. Recent advances in the synthesis and synthetic applications of Betti base (aminoalkylnaphthol) and bis-Betti base derivatives. RSC Advances, 2019, 9: 18467-18497.
[16] Zhao, X. J., Cui, Z. H., Wang, R. L., et al. Synthesis of an electron-rich aniline-containing dye and its dyeing behaviors on silk through a three-component Mannich-type reaction. Chinese Chemical Letters, 2015, 26: 259-262.
[17] Fan, S. J., Ou, Q., Wang, R. L., et al. Study on water-soluble aniline-based dye modification on silk using the Mannich reaction. Journal of Zhejiang Sci-Tech University, 2016, 35(1): 1-8.
[18] Yin, L., Liu, N. P., Cui, Z. H., et al. Study on the selection and effect of aldehydes in the Mannich dyeing method. Journal of Zhejiang Sci-Tech University, 2017, 37(3): 336-342.
[19] Chen, W. G., Gao, P., Jiang, H., et al. A novel reactive dyeing method for silk fibroin with aromatic primary amine-containing dyes based on the Mannich reaction. Dyes and Pigments, 2019, 168: 300-310.
[20] Guo, Q., Chen, W. G., Cui, Z. H., et al. Reactive dyeing of silk using commercial acid dyes based on a three-component Mannich-type reaction. Coloration Technology, 2020, 136: 336-345.
[21] Gao, P., Cui, Z. H., Chen, W. G. Adsorption characteristics of aniline-based dyes for the coloration of silk fibroin. Silk, 2020, 57(5): 1-5.
[22] Cui, Z. H., Gao, P., Zheng, J. H., et al. The selectivity of Mannich-reaction-based modification on amino acid residues in silk fibroin. Dyes and Pigments, 2022, 200: 110100.
[23] Guo Q, Chen W G, Gao P, et al. Synthesis and spectral properties of H acid-containing dyes and their Mannich-type dyeing performances on silk fibroin [J]. Dyes and Pigments, 2022, 204: 110469.
[24] Guo Q, Chen W G, Qi D M, et al. Photostability of Mannich-type dyed silk fibroin with pyrazolone-containing aromatic primary amine dyes [J]. Coloration Technology, 2023: 1-12.
[25] Ohe T, Yoshimura Y. Coloration of polyamide fibers in an aqueous solution by Maillard reaction [J]. Textile Research Journal, 2014, 84 (5): 539-545.
[26] Ohe T, Nakai T, Yoshimura Y. Coloration of different textile fibers using glycerol oxides [J]. Textile Research Journal, 2016, 86: 2216-2224.
[27] Cui L, Yuan J, Wang P, et al. An eco-friendly phosphorylation of wool using Maillard reaction for improving cationic dye absorption [J]. Journal of Cleaner Production, 2018, 178: 611-617.
[28] Cui L, Guan RC, Meng YX, et al. Simultaneous coloration and antibacterial modification of wool fabric with chitosan oligosaccharide via Maillard reaction [J]. Fibers and Polymers, 2023, 24: 1941-1949.
[29] Xie Qiuhua, Jiang Yazhen, Zhang Zhihua, et al. Dye-free wool coloration based on the Maillard reaction [J]. Fine Chemicals, 2023, 40 (5): 1130-1135.
[30] Zhang XY, Sun YF, Qiu T, et al. An eco-friendly and low-temperature dyeing for wool fibers using dihydroxyacetone-induced Maillard reaction [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 680: 132695.
[31] Lim Y J, Lee H K. Synthesis of reactive dye for polypropylene fiber [J]. Journal of the Korean Chemical Society, 1979, 23 (6): 412-416.
[32] Lee H K, Lim Y J, Min K E, et al. Studies on reactive dyes for polypropylene fiber [J]. Journal of the Korean Chemical Society, 1984, 28 (6): 425-432.
[33] Jiang H, Guo G L, Chen W G, et al. Reactive dyeing of synthetic fibers employing dyes containing a diazirine moiety [J]. Dyes and Pigments, 2021, 194: 109555.
[34] Guo Guangluo, Jiang Hua, Cui Zhihua, et al. Synthesis of reactive dyes containing bis-acridine and their dyeing performance on polypropylene [J]. Dyes and Dyeing, 2022, 59 (2): 14-18.
[35] Guo G L, Jiang H, Chai LQ, et al. Design and synthesis of diazirine-containing dyes for polypropylene fiber: A study on the effect of alkyl chain [J]. Coloration Technology, 2022, 138 (5), 551-564.
[36] Wang Y, Zhao T, Bi X, et al. Synthesis of novel carbene dyes and investigation of their dyeing properties and reaction mechanism for various fabrics [J]. Dyes and Pigments, 2024, 221: 111784.
[37] Shi Lulu, Jiang Hua, Xie Xiaokang, et al. Synthesis of carbene-type dyes based on α-phenyl diazoketones and their dyeing performance on spandex [J]. Journal of Zhejiang Sci-Tech University: Natural Science Edition, 2024, 51 (3): 358-368.
[38] Hanna H L. Dyeing of polyurethane elastomeric materials in non-aqueous media [J]. Textile Research Journal, 1975, 45 (7): 573-576.
[39] Mishukova A S, Safonov V V, Apyari V V. One-step dyeing of polyurethane fibers [J]. Fibre Chemistry, 2020, 51 (6): 427-429.
[40] National Archives Administration (Central Archives). China’s Archive Document Heritage List: Volume Five [M]. Beijing: Rong Bao Zhai Press, 2023: 288.
Research Progress in Dyeing Methods Based on Chemical Reactions
JI Zhengfang1,2 JIANG Hua3
( 1. Zhejiang Hongyi Chemical Co., Ltd., Lishui 323700, Zhejiang China; 2. Zhejiang Daoyuan New Mate-rials Co., Ltd., Lishui 323700, Zhejiang China; 3. Engineering Research Center for Eco-Dyeing and Finishing of Textiles, Ministry of Education, Zhejiang Sci-Tech University, Hangzhou 310018, Zhejiang China)
Abstract: Some new reactive dyeing methods developed in recent years based on chemical reactions were introduced, including diazo-tization-coupling reaction, diazotization-nucleophilic substitution reaction, Mannich reaction, Maillard reaction, carbene chemical re-actions, etc. The basic principles, dyeing processes, and dyeing performances of these dyeing methods were given. Their advantages and disadvantages were evaluated. The prospects of these reactive dyeing methods were discussed.
Keywords: reactive dyeing; coupling reaction; Mannich reaction; Maillard reaction; carbene insertion reaction
The "Analysis and Standards" section mainly focuses on the analysis of dyes and dye intermediates, including various chemical analyses, instrumental analysis experiments, dye profiling verification, and product identification, all of which are published in this section.
The "Environmental Protection Technology" section focuses on articles related to the treatment of "three wastes" (wastewater, waste gas, and solid waste) in dyes and dye intermediates, including the introduction and improvement methods of various new and traditional processes, as well as theoretical articles on "three wastes" treatment. Articles on the synthesis of dyes and intermediates with the aim of reducing the "three wastes," as well as articles on reducing the use of auxiliaries, are categorized under "Dyes and Pigments," "Intermediates," and "Auxiliaries and Finishing."
The "Equipment and Control" section reports on various new types of equipment and automation improvements. Under the new situation, the manuscripts received in this section have not reflected the corresponding development status. It is hoped that researchers and production personnel will combine equipment upgrades and modifications to publish relevant discussions.
The "Practice and Communication" section mainly publishes industry articles that are difficult to categorize, such as systematic content of major inspection and exchange activities, exploratory discussions within the industry, and usage methods of new books, new products, and new equipment.
The "News" section focuses on conference notices, new product launches, submission guidelines, and introductions of manufacturers and organizations. Some news items are time-sensitive, and publication will be determined based on specific circumstances.
This journal does not have an online editing and submission system and only accepts submissions via email. All manuscript acceptance, content revisions, and important information collection will be confirmed by email. The journal does not charge any fees for publication, such as page fees, review fees, or postal delivery fees. Unless there are special circumstances, author fees are paid based on the page count of the article within six months after publication, with a standard rate of 40 yuan per page. Articles less than half a page will be counted as half a page, and those exceeding half a page will be counted as one page.
All submissions to the journal are accepted electronically. The submission email is [email protected]. The editorial office's phone number is 024-85869001, WeChat ID: 13002495121, Zhou Xin. If you do not receive a clear response within 5 working days after submitting your manuscript, please directly call the editorial office for an inquiry.

 EN
EN 中文
中文 ES
ES




.jpg)